Top Five Chemical Release Risks In Your School
Newsletter Article


Chemical releases can be scary and can happen anywhere, even in schools. Children are more susceptible to chemical substances in their environment than adults. This increased risk is associated with their smaller physical size, the changes in their organ system functioning and their metabolic capabilities. Because of this, if a child is exposed to a chemical release, they will suffer more significant effects or illness.
Children also spend approximately a third of their lives in school buildings, making it critical that our schools are clean and safe from chemical releases. About 1.3% of all chemical releases happen in schools. This is relatively low but can cause a substantial burden on schools through student illness and costly evacuations.
Five Most Common Chemical Releases in Schools
The five most common chemical releases are natural gas, mercury, carbon monoxide, pepper spray and pool chemicals. This article will examine how these chemical releases are most likely to occur in schools and how to prevent them.
1. Natural Gas (CH4)
Natural gas is the most frequently released chemical in schools. It is a poisonous gas and highly combustible. Leaks can quickly build up in a room, creating the risk of a powerful explosion.
How to detect a Natural Gas Leak in Schools:
- Mercaptans are added to natural gas to give it the odor of rotten eggs, therefore, many gas leaks are discovered by smell. It is common to find small gas leaks after the school has been empty for a few days, such as at the end of breaks. An empty school’s low air movement gives small gas leaks a chance to build up and be detected.
- Listen for a hissing sound; larger leaks will be audible.
- Look for patches of dead vegetation or bubbling around the building. A water puddle that bubbles could be a sign of an underground leak.
What to do if Natural Gas Leaks in Your School:
Follow your school plan, which may have details not listed here.
- Notify the school community that the area should be evacuated immediately if a natural gas leak is detected. Do not flip light switches or use any phones.
- Once you are out of the area, call 9-1-1
- Do not reenter until the all-clear is given by emergency personnel.
Causes of Natural Gas Leaks in Schools:
- Most natural gas leaks are caused by construction or maintenance in the area.
How to Prevent Natural Gas Leaks in Schools:
- Call 811 before anyone digs in the area to prevent gas lines from being damaged.
- Provide repair contractors with an appropriate map of gas lines within the building.
2. Mercury (Hg)
Mercury is the second most frequent chemical release in schools. Historically called quick-silver, mercury is a liquid or gaseous metal at room temperature. It is highly toxic.
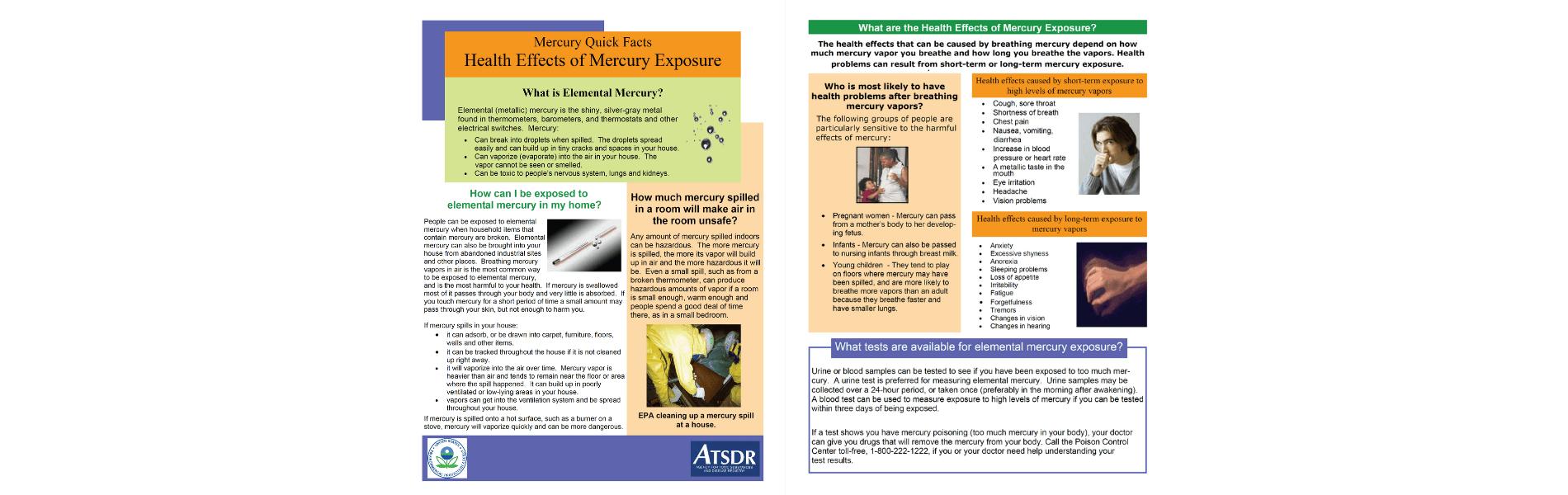
How to detect a Mercury leak in schools:
Mercury is a silvery-white liquid with no odor. So the only way to detect it is to see it. Unfortunately, since the metal is prone to breaking into tiny particles, it is not always easy to see.
What to do if Mercury leaks in your school:
Follow your school plan, which may have details not listed here.
- Don’t touch it or try to clean it up by yourself.
- Leave the room to avoid the vapors. As people leave the room, be sure to not step near the spill so that the mercury is not carried on shoes.
- DO NOT try to clean mercury with a broom or vacuum, as this will increase the vapors in the area.
- If it is a small spill (one thermometer), follow the instructions given by ATSDR Agency for Toxic Substances and Disease Registry.
5. If you are unsure, call 9-1-1
Sources of mercury found in schools:
- Fluorescent light bulbs
- Thermostats
- Thermometers
- Barometers
- Sphygmomanometer for blood pressure measurement
- Mercury oxide or mercury zinc (button style) Batteries
- Containers in the chemical storage room.
- Students bring it into school to show their friends.
How to Prevent mercury leaks in schools:
- Educate staff and students about the dangers of mercury.
- Remove all, or as much as possible, mercury from schools.
- Utilize the Don’t Mess with Mercury resource created by the CDC in partnership with the Agency for Toxic Substances and Disease Registry to help schools prevent spills.
3. Carbon Monoxide (CO)
CO is a colorless, odorless gas caused by incomplete combustion of fuels. When people are exposed to CO, it displaces oxygen in their bodies which can lead to illness or death. Carbon monoxide is the third most frequent chemical release in schools.
How to detect a Carbon Monoxide leak in your school:
CO can only be detected with a CO detector. However, not all states require them in their schools. If your school does not have CO detectors, it is crucial to know the symptoms of CO exposure to identify leaks. CO poisoning causes flu-like symptoms; headache, dizziness, nausea, and unconsciousness. Therefore, teachers and staff need to be the primary source for noticing if a group of individuals is experiencing these symptoms.
Sources of carbon monoxide found in schools:
- Combustion utilities such as boilers, furnaces and water heaters when are poorly maintained or vented.
- Gas stoves in the cafeteria or culinary arts classrooms.
- Exhaust leaking into buses
What to do if carbon monoxide leaks in your school:
- Follow your school plan, which may have details not listed here.
- Notify the school community that the area should be evacuated immediately.
- Once you are out of the area, call 9-1-1
- Do not reenter until the all-clear is given by emergency personnel.
How to prevent carbon monoxide leaks in your school:
- Install carbon monoxide detection as required by the law in your state.
- Identify the presence of any equipment or situation that has the potential to produce carbon monoxide in your district’s buildings.
- Schedule regular inspections and preventive maintenance of all fuel-burning equipment.
- Prohibit vehicles from idling close to school buildings or inside garages.
- Ensure that fuel-powered outdoor equipment is not used indoors or close to the building’s exhaust system.
- Equip the maintenance department with high-quality carbon monoxide sensors.
4. Pepper Spray (C18H27NO3):
This is the fourth most likely chemical to be released in schools. This spray is often used for self-defense and is legal in all 50 states. Pepper spray releases are often the result of students intentionally releasing it in pranks or fights. It can cause burning eyes, difficulty breathing and coughing.
Sources of Pepper Spray found in schools:
- Students bring it to school for protection or as a weapon.
- Safety officers and other staff often carry it.
What to do if Pepper Spray leaks in your school:
Follow your school plan, which may have details not listed here.
- Notify the school community that the area should be evacuated immediately and ensure students know where to go.
- Call 9-1-1 if necessary for students that need treatment.
- For eyes: Immediately apply water to the affected area. Flush eyes, but do not rub. Remove contact lenses.
- For skin: Do not use lotion. Instead, wash the affected area with soap and water, then pat dry.
- Be patient: It could take up to an hour for the effects to subside.
How to prevent pepper spray leaks in your school:
- Teaching students and staff healthy, nonviolent ways to resolve conflicts is the best way to prevent the intentional release of pepper spray.
- Establish rules that pepper spray is not allowed on school grounds.
5. Pool Chemicals
Pool Chemicals are the fifth most likely chemical to be released in schools. Many chemicals are associated with swimming pools, but hypochlorite (Ca(OCl)2) and hydrochloric (muriatic) acid (HCl) are the most common.
Sources of pool chemicals in schools:
If a school maintains a swimming pool, it must also store and use pool chemicals. Most pool chemical releases are caused by human error, incorrectly adding chemicals to pools and equipment failure.
What to do if pool chemicals release in your school:
Follow your school plan, which may have details not listed here.
- Stand up (chlorine gas is heavy and will be down low.) and move to fresh air.
- Wash face and skin with fresh water. Eyes need to be rinsed for a full 15 minutes to limit the damage.
- Seek medical attention for any symptoms lasting longer than 3 minutes.
- It may be necessary to evacuate the school community as the fumes will travel.
How to prevent pool chemical releases in your school:
- Training for pool operators.
- Routine maintenance of pool equipment.
- Proper storage of chemicals.
- Dispose of all chemicals according to hazardous waste laws.
In conclusion, it is crucial for schools to be aware of the top five chemical release risks and take proactive measures to ensure the safety of students and staff. By addressing the risks associated with natural gas, mercury, carbon monoxide, pepper spray, and pool chemicals, schools can mitigate potential incidents and protect the well-being of everyone in your district.
Anderson, Ayana R., et al. “Hazardous Chemical Releases Occurring in School Settings, 14 States, 2008-2013.” Journal of Environmental Health (2017): E1-E7. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5812020/#R11>.
Registry, Agency for Toxic Substances and Disease. Why Are Children Often Especially Susceptible to the Adverse Effects of Environmental Toxicants? 15 February 2012. course. 16 May 2023. <https://www.atsdr.cdc.gov/csem/pediatric-environmental-health/why_children.html>.
Sadrizadeh, Sasan, et al. “Indoor air quality and health in schools: A critical review for developing the roadmap for the future school environment.” Journal of Building Engineering 57 (2022). Web site. <https://www.sciencedirect.com/science/article/pii/S2352710222009202>.
Want help managing your district’s chemical safety?
Our Chemical Hygiene and Laboratory Safety Program helps districts maintain safe laboratories and prepare students and staff for how to respond to a chemical accident or emergency.
